An estimate of LVEF is of great importance in patients admitted to the ICU since decreased LVEF directly impacts on clinical management. Several parameters are readily available to help intensivists determine whether a patient is in shock, such as physical examination, plasma lactate and SvO2. However, none of these parameters in themselves are diagnostic as to the cause of shock and therefore ultrasound is frequently used to image the heart with the goal to identify patients suffering from heart failure as a cause for shock.(ref 1)
When performing cardiac ultrasound several measurements can be performed to roughly estimate LVEF, such as ‘eyeballing’, the Simpson method or measurement of mitral annular plane systolic excursion (MAPSE). Clinicians often ‘eyeball’ the left ventricle in order to estimate the LVEF. Eyeballing has several important pitfalls since it is known that although it may be a reliable way of estimating LVEF in trained and experienced observers, such as cardiologists, it is quite unreliable in inexperienced observers, which intensivists frequently are. Eyeballing is not only operator dependent, it is hard to repeat over time to monitor treatment response and it requires at least two views of the heart.(ref 1)
E-point septal separation (EPSS):
Assessing LVEF by using the E-point septal separation (EPSS) method has shown to be a reliable way of objectivising (severely) reduced LVEF (<50%).[2,3.8-10] EPSS is a quick and easy measurement which we can use to rapidly identify those patients in whom the ejection fraction is significantly reduced and who may benefit from inotropic treatment. It is a quick and rather dirty method to get an indication of whether significantly reduced LVEF is present, nothing more and nothing less.

Assessment of Cardiac Output:
Cardiac output assessment is an essential tool in critical care. One of the available noninvasive options to measure cardiac output is by using ultrasound. The most widely accepted and easily applicable method is to measure the left ventricular outflow tract (LVOT) diameter and combine this with flow velocity estimation using pulsed-wave Doppler. The velocity time integral (VTI) is the stroke distance of blood during systole (cm) combined with the LVOT cross-sectional area (cm2). The LVOT diameter (cm) is measured at the insertion of the aortic valves at systole in the parasternal long axis view and the aortic valve area is calculated by the formula Π x (diameter LVOT/2)2 /4. Finally, to obtain the cardiac output this product is multiplied by the heart rate.Measurement inaccuracies in measuring the LVOT diameter cause great variation in cardiac output results given that any error in diameter measurement will be squared when computing the cross-sectional
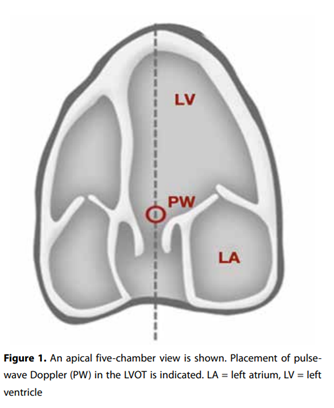
area. This results in overestimation or underestimation of the stroke volume. However, the LVOT diameter is very stable throughout the cardiac cycle which means aortic VTI variations are proportional to stroke volume variation, so that the VTI is an excellent surrogate marker for stroke volume and cardiac output.From the A4C view, the apical five-chamber view is obtained by tilting the ultrasound beam anteriorly until the LVOT, aortic valve and the proximal ascending aorta come into view (figure 1). If this view is obtained, the pulse-wave Doppler is placed in the LVOT just proximal to the aortic valve. The Doppler wave formed is frozen and the downsloping envelope
is traced (figure 2). Finally, the LVOT VTI may be measured in cardiac output assessment on your ultrasound machine. VTI in normal adults at rest is usually in the range of 18-22 cm, values below this reflect a decreased cardiac output.(ref 2)

References:
1. POCUS series: E-point septal separation, a quick assessment of reduced left ventricular ejection fraction in a POCUS setting
S.C. Boon, J.E. López Matta, C.V. Elzo Kraemer, P.R. Tuinman, D.J. van Westerloo. Neth J Crit Care May 2020;28(3): 139 - 141 (https://njcc.nl/sites/nvic.nl/files/pdf/original-article1_2.pdf)
2. POCUS series: The use of velocity time integral in assessing cardiac output and fluid responsiveness
J.E. López Matta, C.V. Elzo Kraemer, P.R. Tuinman, D.J. van Westerloo. Neth J Crit Care September 2019;27(5): 196 - 8 (https://njcc.nl/sites/nvic.nl/files/pdf/original-article1_0.pdf)
Chief-editors
Pieter Roel Tuinman, MD, PhD, intensivist-epidemiologist
David van Westerloo, MD, PhD, intensivist
Editors:
Carlos Elzo Kraemer, MD, intensivist
Jorge Lopez Matta, MD, intensivist
Paul Wijnandts, MD, intensivist
Jasper Smit, MD, PhD student
Mark Haaksma, MD, PhD student
Micah Heldeweg, MD, PhD student
Annemijn Jonkman, technical physician, PhD student
Heder de Vries, MD, PhD student
Contact
Department of Intensive Care Medicine
Amsterdam University Medical Centres, Vrije Universiteit Amsterdam
Room ZH - 7D-166
De Boelelaan 1117
1081 HV Amsterdam, The Netherlands
prtuinman@hotmail.com
Department of Intensive Care Medicine
Leiden University Medical Center (LUMC)
