Lung ultrasonography and COVID-19:
POCUS has shown to be a valuable tool in patients with COVID-19, since it can allow the concomitant execution of clinical examination and lung imaging at the bedside by the same physician, without transportation of the patient and use of radiation. However, in patients with infection in general, and especially COVID-19, there are some important rules to adhere to:
- use protective gear (e.g. FFP2 mask, gloves, etc) and disinfect your hands after each examination in the emergency department. In the ICU, depending on the availability of protective gear, this must be adjusted accordingly.
- use a standard and succinct protocol, e.g. the BLUE-protocol or 12-zone approach; focus on the relevant, but avoid tunnel vision; Shorter exposure to an infected patient reduces the chances of you getting infected
- clean/disinfect your machine (see for example: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2) (1) see Figure A.
Figure A. Cleaning and disinfecting procedure (1)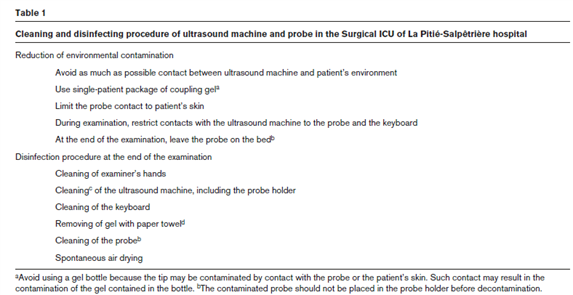
Findings of lung ultrasonography in COVID-19 pneumonia patients:
Characteristic findings of lung ultrasound in COVID-19 pneumonia patients are shown in Table 1. A 12-zone approach was used in these patients (2). The observed patterns occurred across a continuum from mild to severe disease. The findings of lung ultrasonography are related to the stage of the disease.
Table 1. Characteristic ultrasonographic features of COVID-19 pneumonia patients (n=20) and their CT correlate (adapted and modified from Peng et al Intensive Care Med 2020 March 12) (3).
|
Lung ultrasound |
Chest CT |
|
Thickened pleural line |
Thickened pleura |
|
B-lines (discrete, multifocal, confluent) |
Ground glass |
|
Confluent B-lines |
Pulmonary infiltrating shadow(?) |
|
Small (centomeric) consolidations |
Subpleural consilidations |
|
Non-translobar and translobar consolidation |
Translobar consolidation |
|
Multilobar distribution |
>2 lobes affected |
|
Pleural effusion is rare |
Pleural effusion is rare |
|
Focal B-lines is main feature in the early stage and in mild infection; AIS is first feature of progressive disease; pleural thickening with uneven B-lines can be seen in patients with fibrosis |
A typical or negative lung CT in very early stage and mild infection; Then diffuse scattered or ground glass shadows with progression; further lung consolidation; later stage signs of fibrosis |
It was shown by our group (Haaksma et al. ERJ Open Research 2020) that SARS CoV-2 results in significant, but not specific, ultrasound changes, with decreased lung sliding, thickening of the pleura and a B-profile being the most observed. With time, a thickened and irregular pleura, C-profile and pleural effusion become more common findings.
Two main patterns were found by colleagues in northern Italy, a diffuse interstitial syndrome also in anterior fields (potential PEEP responders) and another with clean anterior fields but posterior consolidations (potential not so responder to Peep but more to proning) (personal communication).
Of note, chest CT is required to further quantify the stage of the disease, especially when pulmonary fibrosis or a superinfection with aspergillus is suspected. However, Heldeweg et al (submitted for publication) showed that the correlation between LUSI and CTSI was strong (r=0.795), with an overall 15% bias, and limits of agreement ranging -40 to 9.7. Concordance between changes in sequentially measured LUSI and CTSI was 81%. In the univariate model, high involvement on LUSI and CTSI were associated with a composite endpoint. In the multivariate model, LUSI was the only remaining independent predictor.
Indications:
Lung ultrasonography can be used to:
- Diagnose COVID-19 pneumonia (e.g. using the BLUE-protocol, Figure 1 (4) or a more extensive 12 zone approach) (2)
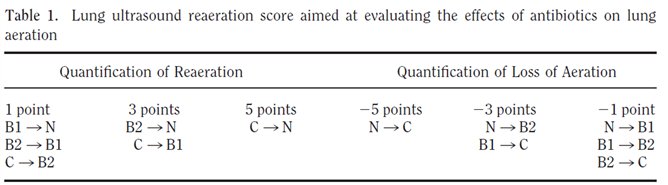
- To monitor progression of the disease using the lung ultrasound score (12 zone approach)(5, 6); Figure 2 and 3.
- To monitor lung recruitment manoeuvres using the re-aeration score (12 zone approach)(5, 7); Figure 2 and 3.
- To guide and monitor response to PEEP increase or to prone position, e.g. by using the re-aeration score (12 zone approach)(5, 7); Figure 2 and 3.
The more extensive ultrasound ABCD-approach (compassing ultrasound of heart, lung, diaphragm and upper abdomen) can be used for making decisions related to weaning the patient from the ventilator (8). Because most COVID-19 patients will have a neutral or negative cumulative fluid balance, ultrasound of the abdomen is considered to be less relevant in these patients.
Figure 1: The BLUE-protocol (4)
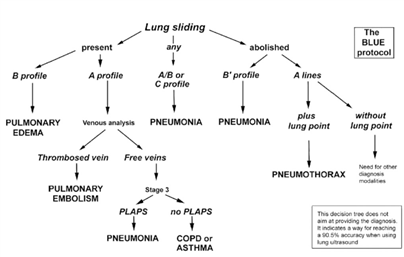
Figure 2: The lung ultrasound score (6)
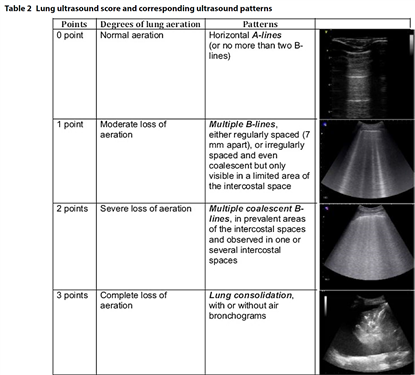
Figure 3. Loss of aeration of > 400 ml was detected by a lung ultrasound score of <-10; Reaeration of > 400 mL was detected by a lung ultrasound score of ≥ 5 (7).
For an overview see also our publication (ref 10): https://njcc.nl/sites/nvic.nl/files/pdf/review1_4.pdf
References:
- Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: Bedside lung ultrasound in critical care practice. Crit Care. 2007;11(1):205.
- Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40(7):2064-72.
- Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study G. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020.
- Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-25.
- Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183(3):341-7.
- Mayo PH, Copetti R, Feller-Kopman D, Mathis G, Maury E, Mongodi S, et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019;45(9):1200-11.
- Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38(1):84-92.
- Tuinman PR, Jonkman AH, Dres M, Shi ZH, Goligher EC, Goffi A, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med. 2020;46(4):594-605.
- Haaksma et al. Lung ultrasound findings in patients with novel SARS-COV-2. ERJ Open Research 2020.
- A.X. Elzo Kraemer, S.L. Staal, J.E. López Matta, M.E. Haaksma, M.L. Heldeweg, J.M. Smit, P.R. Tuinman, D.J. van Westerloo, C.V. Elzo Kraemer. POCUS series: point-of-care lung ultrasound in patients with COVID-19. Neth J Crit Care July 2020;28(4): 162 - 4
Chief-editors
Pieter Roel Tuinman, MD, PhD, intensivist-epidemiologist
David van Westerloo, MD, PhD, intensivist
Editors:
Carlos Elzo Kraemer, MD, intensivist
Jorge Lopez Matta, MD, intensivist
Paul Wijnandts, MD, intensivist
Jasper Smit, MD, PhD student
Mark Haaksma, MD, PhD student
Micah Heldeweg, MD, PhD student
Annemijn Jonkman, technical physician, PhD student
Heder de Vries, MD, PhD student
Contact
Department of Intensive Care Medicine
Amsterdam University Medical Centres, Vrije Universiteit Amsterdam
Room ZH - 7D-166
De Boelelaan 1117
1081 HV Amsterdam, The Netherlands
prtuinman@hotmail.com
Department of Intensive Care Medicine
Leiden University Medical Center (LUMC)
